
CASE NOTES
Screwworm – risk and recognition
Peter James 1 and Peter Green 2
1 Queensland Alliance for Agriculture and Food Innovation (QAAFI)
University of Queensland
2 Queensland Department of Employment, Economic Development and Innovation (DEEDI)
Posted Flock & Herd March 2011
Introduction
The Old World screw-worm, Chrysomya bezziana (OWS) is considered to be the most serious exotic insect pest threatening Australia's livestock industries and is endemic in a number of our closest neighbouring countries (Fig 1(a)). Habitat conditions over much of tropical and subtropical Australia are favourable for the establishment of OWS (Fig 1(b)) and the risk of an incursion may have increased in recent years with the growing live animal export trade from northern Australia and increased movement of people and animals to Australia's northern shores. Incursion and establishment of screwworm in Australia's extensive livestock industries, particularly the northern beef industry, would have major economic and welfare impacts. The total cost of the establishment of OWS in Australia, based on 2003 prices, was estimated to be approximately $400 million per year to the northern cattle industry alone once screw worm fly reached its ecological limits (Animal Health Australia 2004). All warm blooded animals including Australian native fauna and occasionally humans are potential hosts and there are significant animal welfare as well as economic imperatives to prevention and eradication of incursions.
Figure 1. (a) Current world distribution of old and new world
screwworm; (b) potential C. bezziana distribution in Australia
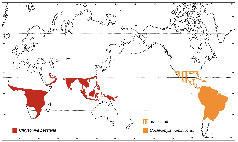
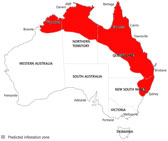
Source: AUSTVETPLAN 2007
The OWS is an obligate parasite of warm-blooded animals, the females laying batches of eggs at the edge of wounds. Virtually any wounds whether caused accidentally (for example tick bites, thorn scratches or fighting wounds) or through management practices such as castration, de-horning, shearing and tail docking are at risk. The navels of the new born and the vulval region of their mothers, particularly when injured while giving birth, are highly susceptible to strike. Mucous membranes associated with natural body openings such as the nostrils and sinuses, the eye orbits, mouth, ears and vagina can also be struck. During the 6-7 days of feeding, the larvae burrow deeply into the host tissue, destroying muscle and causing severe blood loss. Reduction in productivity, maiming or death can result from screw-worm infestations.
Figure 2: Life stages of C. bezziana

MJR Hall, Natural History Museum, London
Although OWS is considered to present the major risk because of its geographic proximity to Australia, other similar myiasis flies, for example the New World Screwworm (NWS) (Cochliomya hominivorax), endemic to South America and previously central America and the southern United Stages (Fig 1), and Wolfartia magnifica, present in southern Europe, Russia, the Middle East and China, also present a risk. A previous detection of screwworms in Australia, in a woman returning from South America, was NWS (Searson et al. 1992).
Surveillance
An active surveillance program is in place in Australia, particularly in high risk areas such as the northern coast line of Australia and Torres Strait, livestock export ports and northern export abattoirs to try and ensure early detection of any screwworm incursion into Australia. Surveillance includes fly trapping, quarantine officer inspection of livestock export vessels and monitoring of sentinel herds in northern Australia. Clearly a major part of the surveillance program relies on submission of suspicious samples collected from struck animals by livestock and pet owners, veterinary practitioners and animal health officers. This paper gives an overview of the gross morphological features of OWS life stages which may help to enable a preliminary assessment. However, identification of OWS larvae and adults and discrimination from endemic Calliphorid species is a skilled task and where there is considered to be a risk, specimens should be collected, preserved and labelled as described below and forwarded to State Department of Primary Industries, Biosecurity or AQIS laboratories for specialist examination.
For more detailed information on the identification of screwworm strike and descriptions of the different life stages of OWS, see Spradbery (2002) A Manual for the Diagnosis of Screw-worm Fly and the AUSTVETPLAN Screw-worm Disease Strategy, both readily accessible on the web. In addition, a Lucid key to aid the identification of myiasis-causing fly larvae is available from the Museum of Natural History, London website (Adams Z, Smith L and Hall MJR) at www.nhm.ac.uk. A poster on screwworm biology available from the Animal Health Australia site (www.animalhealthaustralia.com.au ) also provides good information and pictures to aid identification.
Adult flies
The adult screwworm fly is a medium sized shiny blue-green blowfly with some darkening to the back of the abdominal segments. It belongs to the family Calliphoridae, the same group as commonly known endemic blowflies. It is closely related to the secondary flystrike species Chrysomya ruffiface, which is responsible for the 'hairy maggots' sometimes found in sheep flystrikes and which is widely distributed through most of Australia. However whereas C. rufficacies has a whitish or silverish face covered with fine black hair the adult OWS fly has a yellowish 'face' with soft fine yellow hairs. In addition, the anterior spiracle is pale or white in C. rufifacies whereas it is dark brown or dark orange in OWS.
OWS is also closely related and very similar in appearance to Chrysomya megacephala, the oriental latrine fly, which occurs in northern Australia and can also sometimes lay eggs in wounds, usually as a secondary coloniser. Distinguishing adult C. megacephala from C. bezziana is more difficult and may need specialist input. C. bezziana male flies do not have a boundary dividing the upper and lower eye facets, while C. megacephala do. Females can be distinguished by the form of the frontal stripe between the eyes. The frontal stripe of C. bezziana is parallel-sided, whereas C. megacephala has a frontal stripe that is broader in the middle and the sides not parallel.
Figure 3: Chrysomya bezziana (yellow 'face'), C. ruffifacies (white-silver 'face') and C. megacephala



Screwworm strikes and maggots on animals
Although structured surveillance programs often incorporate trapping of adult flies, primary detection of an incursion at the farm level is most likely to be through maggots found in wounds.
OWS eggs are white in colour and laid on the edge of wounds, all oriented in the same direction in a pattern like roof-tiles. The screw worm maggots are whitish to cream in colour and have bands of dark, backward facing spines growing on each body segment giving the larvae a screw-like appearance. There are three instars (Fig 2), the first and second lasting for about 24 h each and the third feeding for 3-5 days, giving a total time in the wound of about 6-7 days.
Figure 4: Typical
pocket-like OWS wound with maggots feeding head down
and tightly packed in a
pulsating mass

The maggots form typical pocket-like wounds, with the larvae feeding tightly packed and head down in the wound with the posterior segment and rear spiracles obvious.
Even in the early stages of a screwworm strike, before the larvae are readily apparent, there will be a characteristic pungent foul smell associated with the wound. Clinical signs are similar to those seen in flystrike. Animals will lick or bite at the wound and show general restlessness. They may develop a fever, lose appetite and where strikes do not resolve death may result. Screwworm wounds can extend deeply into subcutaneous soft tissue and muscle and expansion of wounds into body cavities is common. This can sometimes lead to peritonitis in the case of navel infestation, sinusitis following infestation of dehorning wounds or pleuritis following thoracic strikes.
Often diagnosis can be complicated by secondary infestations by other fly species. Usually these larvae feed towards the surface of the wounds whereas OWS tends to burrow more deeply. The best way to identify screwworm is through the examination of third instar larvae. As noted above, OWS and NWS larvae have dark spines growing on each body segment. This is distinct from the tubercules present on the 'hairy maggots' of the secondary flystrike species C. ruffifacies and C. varipes (Fig 5) and different from the smooth maggots of L. cuprina and brown blowfly species (Calliphora spp.) that can also sometimes infest wounds (Fig 5).
Although Fig. 5 gives an idea of the general comparative morphology of screwworm larvae and some of the species commonly found in wounds, many other species can also occur. Accurate identification requires examination of a range of characters, such as structure of the rear and anterior spiracles, spine morphology and configuration, tracheal trunk pigmentation and, in some instances, molecular sequence data. Identification is a specialist task and all suspicious larvae removed from wounds should be forwarded for expert identification
Figure 5: Larvae of
OWS showing characteristic spines, which give a screw like appearance,
the
'hairy' maggot fly (Chrysomya rufifacies) showing tubercules that give
the
hairy appearance and 'smooth' maggots of L. cuprina.



Sukontason K. et al. Parasitol Res (2010) 106: 641
Collection for identification
The diagnostic characters given above are indicative at best and suspect larvae collected from a wound should be submitted for expert identification. Mature third instar larvae are best for identification so select the largest larvae. Secondary infestation is common and there may be more than one species of larvae present in the wound. Select the larvae from the deepest part of the wound as larvae from the surface or periphery of the wound are more likely to be secondary species.
Larvae can be preserved in 70% ethanol or methanol. However, direct immersion of live larvae into the preservation fluids causes contraction as they die and makes it more difficult to see some diagnostic features. Good quality specimens can be obtained if larvae are first killed by immersion in near-boiling water for 15-30 seconds. Hot water can be taken to the collection site in a thermos flask or it can be prepared on site, using for example a portable gas heater or a water heater operated from a car cigarette lighter. After killing, larvae should be transferred to 80% ethanol or methanol for storage until they can be examined.
As adult flies are usually easier to identify than larvae, it can assist to rear some larvae through to adult flies. Large larvae should be placed in a jar or vial filled with sand, vermiculate or paper and with a perforated lid to allow oxygen exchange and retained at room temperature. Allow the flies to hatch from the puparium and to develop full rigidity and colour before killing them by placing in a freezer for at least an hour. Note that this definitely should not be done if the flies cannot be securely contained and especially if it is considered that there is a significant probability that the species in question might be screwworm.
Full details of the property name and number where samples were collected, date of collection, number or identification of struck animals, site of the wound, and collectors name and contact details should be recorded, desirably on a label attached to the specimen container.
Screwworm research in DEEDI
In recent years we have been conducting research related to screwworm detection and containment in three main areas:
Better trapping systems for surveillance
An improved screw-worm fly trap, LuciTrap with Bezzilure-2, was developed which attracts more screw-worm flies and less other flies than previous trapping systems. The new system will improve screw-worm fly surveillance by providing more sensitive detection of an incursion (Urech et al. in press).
More sensitive detection of screwworm flies in trap catches
Surveillance trap catches have traditionally been screened by sorting through the trapped specimens and microscopically examining suspect specimens for morphological diagnostic characters. However, this is laborious and difficult, especially when trap catches are large and the condition of the specimens poor. A PCR based method with sensitivity of 1 OWS in 1000 non-target species was developed and will assist more rapid and accurate processing of trap catches (Jarrett et al. 2010)
Chemical treatment and containment in the event of an incursion
The AUSVETPLAN screwworm preparedness plan indicates a two stage strategy in the event of a screwworm incursion consisting of containment with chemical treatments and eradication using sterile male release (with complementary use of chemicals). Although only one chemical is currently registered for use against screwworm in Australia, in the event of an incursion an Emergency Use Off-Label Permit could be approved for products registered for animal application and with known efficacy against screwworm. Chemicals that could be used for screwworm containment in Australia were reviewed (James et al. 2005) and we are currently planning a project to provide the further efficacy data required.
References
- AUSTVETPLAN Disease Strategy, Screw-worm fly. Version 3.0 (2007) www.animalhealth.com.au
- James, PJ, Green PE, Urech R and Spradbery, JP (2005). Chemicals for control of the Old World screw-worm fly Chrysomya bezziana in Australia. Animal Health Australia 40pp www.animalhealthaustralia.com.au
- Jarrett S, Morgan JAT, Wlodek BM, Brown GW, Urech R, Green PE and Lew-Tabor AE (2010). Specific detection of the Old World screwworm fly, Chrysomya bezziana, in bulk fly trap catches using real-time PCR. Medical and Veterinary Entomology 24, 227-235
- Searson J, Sanders L, Davis G, Tweddle N and Thornber P (1992) Screwworm fly myiasis in an overseas traveller – case report. Communicable Disease. Intelligence 16, 239-40.
- Spradberry JP (2002). A manual for diagnosis of screw-worm fly. Department of Agriculture Fisheries and Forestry Australia, 62pp. www.animalhealthaustralia.com.au
- Urech R, Green PE, Brown GW, Spradbery JP Tozer RS Mayer DG and Tack Kan Y. Field assessment of synthetic attractants and traps for the Old World screw-worm fly, Chrysomya bezziana Veterinary Parasitology (in press)