
CASE NOTES
DEVELOPMENTAL DUPLICATIONS (DD) IN ANGUS CALVES
Laurence Denholm, Principal Policy Analyst, Trade and Investment NSW and Lisa Martin, District Veterinarian, New England Livestock Health and Pest Authority (LHPA)
Posted Flock & Herd 4 December 2013
Introduction
In December 2011 we reported an increased incidence of polymelia (notomelia, cephalomelia and pygomelia) in Angus cattle which appeared to be transmitted as a heritable disorder. ( See Flock and Herd article www.flockandherd.net.au )
Here we present an update on our more recent work in this heritable syndrome.

THE GENETIC BASIS OF DEVELOPMENTAL DUPLICATIONS
In August 2013 a new autosomal (Chromosome 26) recessive mutation was identified in Angus cattle with polymelia by Prof. Jonathan Beever of the University of Illinois at Urbana, USA, using samples from Australian calves with polymelia that we sent to him in 2011 and 2013. The mutation causes a single amino-acid substitution at a locus that has been conserved without change throughout the evolution of vertebrate and non-vertebrate animals.
As a result of the research described below which demonstrated that polymelia is just one of several forms of developmental duplication caused by this same mutation, the name of this syndrome was changed to "Developmental Duplications" (DD).
Angus Australia, the American Angus Association and Angus breed societies in other countries have declared DD to be a recognized genetic condition in the Angus breed.
The DD allele is transmitted in Angus and Angus infused breeds (Brangus, Black Simmental,etc.) by descendents of a 1977 born US AI sire, Ken Caryl Mr Angus 8017. Almost all DD carriers in Australia today are however descendants of a fifth generation descendent of this 1977 bull, the influential US Angus sire, B/R New Design 036 born in 1990.
Sporadic cases of polymelia have been reported in other breeds including Bos indicus breeds but it is not yet known whether these cases result from the same mutation. One Angus calf with notomelia that does not carry the DD mutation has been identified in NSW, so phenocopies do occur, as is expected.
THE ECONOMIC SIGNIFICANCE OF DEVELOPMENTAL DUPLICATIONS
The economic significance of DD lies partly in the high incidence of dystocia with polymelic and conjoined calves, the high mortality rate in these calves and the cost of the surgical amputations that are necessary in many surviving cases. However, the DD genotyping data also suggests that a significant proportion of DD homozygotes are "missing" from the population - more than would be expected from the reporting rate for DD phenotypes in neonatal calves. This suggests there may be significant embryonic mortality in DD, as reported with similar syndromes in other species.
An analysis of the Angus Australia database has shown that more than 15% of currently registered Angus cattle carry the DD mutation (GeneProb). This analysis also showed that the carrier frequency has been rising rapidly in recent years with the widespread use of AI sires that are sons and grandsons of B/R New Design 036. Without detection of the mutation it is estimated that the DD carrier frequency would have reached 25% in the next decade. Based on the genotyping data, the mortality in Australia from all of the DD phenotypes described below (including embryonic mortality) was estimated to be about 3,000 per annum and rising rapidly towards 10,000 per annum over the next decade. Application of the DNA diagnostic test for identification of DD carriers will however now counter this increase.
With more than 36,000 DNA tests for DD conducted since September 2013, it is now well established that the abnormal phenotypes caused by the DD mutation are transmitted with incomplete penetrance and variable expressivity. Penetrance of the DD phenotype is estimated to be 54%, with 46% of the expected number of homozygotes actually present in the population as apparently normal individuals, although some of these animals are likely to have sub-clinical DD lesions and develop clinical signs at a later time.
Commercial DNA diagnostic testing for the DD mutation is now available through the University of Queensland Animal Genetics Laboratory and Zoetis Australia. DNA testing can be done on tail hair, EDTA blood or tissue samples. Tail hair is preferred.
The following genotype nomenclature has been adopted in Australia.
DDF Free - Animal is a non-carrier based on a DNA test result
DDFU Free Untested - Animal is expected to be a non-carrier based on its recorded pedigree
DDC Carrier - Animal is heterozygous for DD based on a DNA test result
DDA Affected - Animal is homozygous for DD based on a DNA test result. However, due to incomplete penetrance of the DD phenotype, a DDA animal may or may not express clinical signs of DD. Many DDA animals appear to be normal.
DD__% - Probability% that the animal is a DD carrier, calculated by "GeneProb" software from the animal's BreedPlan recorded pedigree
Although the DD mutation was identified initially in calves with polymelia, we had suspected some association between polymelia and conjoined twins and had included a DNA sample from a calf with a partial conjoined "twin" (caudal axial duplication) with the polymelia DNA samples sent to Prof. Beever in 2013. This conjoined calf tested homozygous for the DD mutation.
Based on a literature review of limb duplications and conjoining, we began to suspect that the phenotypes associated with the DD mutation would be much wider than just polymelia and conjoining. With support from Angus Australia and using the DNA test for DD which became available at the Animal Genetics Laboratory of the University of Queensland in September 2013 under license from Prof. Beever, we began a survey of abnormal calves with high DD risk pedigrees and likely phenotypes. Within a few weeks a wide range of new DD phenotypes was identified.
THE EMBRYOLOGICAL BASIS FOR DD
Based on this work, it is now clear that DD is a fundamental defect of embryonic neurulation involving both cranial and spinal dysraphism. (In cattle, neurulation commences before implantation of the embryo in the uterine wall and is complete by the fourth week.)
The wide range of spinal and cranial dysraphism related abnormalities that has now been identified in DDA animals, either together in the same animal or as so-called "isolated phenotypes", has also been reported in dysraphic syndromes of other species.
THE MOLECULAR BASIS OF DD
Nothing is currently known about the function of the protein coded by the DD gene but it is likely to be a regulator of gene transcription in cell-cell signalling during neurulation. The DD gene is not a vertebrate homolog of the slimb gene that is involved in limb duplication in Drosophila melanogaster. The DD and slimb proteins do however have some common features.
PHENOTYPIC PRESENTATIONS OF DD
The clinical presentations of the DD phenotype identified to date include:
- Polymelia: Newborn calves and older animals with one or more extra legs (forelegs and/or hindlegs) projecting from the head or body, usually along or close to the dorsal midline for extra forelimbs and between the normal hindlimbs for extra hindlimbs. The extra leg may have normal anatomy or duplications such as polydactyly within the leg or it may be syndactyl. Proximal bones, particularly the scapula, may be vestigial only. There can be fusion of the bones across proximal joints. The extra legs vary in size and may or may not grow in proportion to the host calf. Dystocia is very common.
- Axial Duplication: Newborn calves with an acephalic conjoined "twin". There may or may not be one or more legs projecting from the conjoined "twin". In some cases the parasitic "twin" is just a mass of tissue on the backline or side of the host calf, but well formed long bones can usually be palpated within this mass. Detection of the axial duplication may require careful dissection, particularly when the duplicated vertebral column is fused along the dorsal margin of the host vertebrae (ie. a rachipagus conjoined "twin"), but other cases have a duplicated caudal vertebral column with pelvis and hindlegs projecting outwards and caudally from the vertebral column of the host. Some of these cases survive but many die in the neonatal period. Serious dystocia is usual in these cases.

- Intradural Embryogenic Teratomas: Mature well differentiated intradural teratomas, located along or close to the dorsal midline and often associated with spina bifida. The teratoma may be overlaid with a substantial fat deposit or a lipoma. The clinical presentation is a congenital soft tissue mass on the dorsal midline, with or without signs of spinal cord dysfunction due to cord tethering or compression. These teratomas usually contain mature cartilage nodules, skin, muscle and adipose tissue. Some contain one or more glandular cysts. DD teratomas occur at either end of the developing neural tube and are associated with duplication or division of the host CNS. In the calf at term, the teratoma is therefore located in the occipital or sacral area.


However, these teratomas can also occur near the thoraco-lumbar junction (in close proximity to the site in the embryo of the organising node of the primitive streak). Some thoraclumbar teratomas are embedded in the dorsal process of a host vertebra. All of these teratomas have a substantial neural connection to the host CNS. This neural connection can be a duplication/division in the brainstem, a duplication/division of the terminal spinal cord in the sacrum or an abnormal nerve root arising from the dorsal surface of the spinal cord, often associated with an area of diastematomyelia or diplomyelia and syringomyelia in the host cord. Teratomas linked to the mid-brain can be entirely intracranial, partly intracranial through an abnormal opening in the occipital bone or entirely extracranial and linked to the mid-brain by a nerve passing through an abnormal foramen in the occipital bone. In one DDA calf we observed an intracranial neural tumour that did not appear to be a mature teratoma, This solid glistening white tumour was located in the arachnoid layer of the meninges overlying the occipital lobes and cerebellum. It had a strong neural connection to the brainstem, as seen with the teratomas.


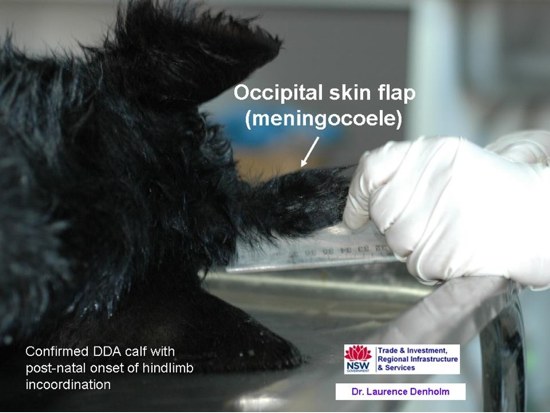
- Dermoid cysts and occipital skin flaps: These are found in similar locations to the teratomas. Dermoids are small nodules (0.5 to 1.5 cm in diameter) and are usually intradural, although they may become extradural with subsequent development. Anterior dermoids may be extracranial or intracranial and caudal dermoids may be extramedullary or intramedullary. The intradural dermoids have a glistening surface and look like a large ganglion externally. Transection reveals a cystic space filled with necrotic fluid or yellow-green cheesy material and black hairs growing internally from the epithelial lining. These dermoids are also neurally linked to the midbrain or spinal cord. Dermoids are considered by some to be degenerate embryogenic teratomas. Some dermoids in DD are only detected at necropsy, but others are located on the occipital bone, near the occipital protruberance, at the base of an endothelial lined flap-like pouch of skin which projects from the head like a third ear, and these dermoids can be detected as a nodule by palpation through the skin in the live calf. These occipital skin flaps are common in DDA cases, as an isolated lesion or in combination with other DD lesions. One 10 day old DDA calf with post-natal onset of progressive hindlimb incoordination was found at necropsy to have a dermoid cyst in an occipital skin flap as well as two spinal teratomas, one sacral and the other in the dorsal process of the second last thoracic vertebra.

- Lipomas: In some DDA cases, there is a lipoma overlaying the embryogenic teratoma or dermoid cyst. These lipomas can be quite large and can look like a beret hat on the live animal's head. Careful dissection is required to locate the teratoma or dermoid in the lipoma, on the surface of the occipital bone near the midline, close to the occipital protruberance and adjacent to an abnormal occipital foramen. Others however may be intracranial.


- Craniofacial dysmorphogenesis: Mild to extremely severe facial dysmorphogenesis secondary to abnormal brain development occurs in DDA calves. Mild cases involve shortening and upwards deviation of the maxillae with mandibular overreaching. The mandibles are normal in milder cases but in more severe cases there can be crowding of the teeth. More severe cases also have a cleft palate and defects of the muzzle. Ocular dysplasia, unilateral or bilateral, is common in these cases, with micropthalmia, fusion of the eyelid or conjunctiva to the cornea, narrow palpebral fissures, hypoplasia of the oculomotor muscles, liquifaction of the lens and failed development of the optic foramen, sometimes with the retinal surface of the eye directly apposed to the brain. An asymmetric defect of the sphenoid bone with projection into the cranial vault is also commonly seen. The similarity of these craniofacial and ocular lesions to those reported in pestivirus infection suggests that such cases should be screened for pestivirus antigen and antibody unless there are other signs of DD not associated with BVDV. The brain developmental defects in these cases include pseudoholoprosencephalon with internal hydrocephalus and Chiari malformation. The cerebral defects are a consequence of the brainstem duplication.


- Craniofacial duplications: More severe craniofacial dysmorphogenesis in DD results in craniofacial duplication - diprosopus ("two faces") and possibly dicephalon ("two heads"). An Angus calf with a complex facial duplication (a third eye in the centre of the face and a duplicate muzzle inserted between the two sides of the normal face) has tested as DDA. Another diprosopus case was reported from the main indicator herd for DD in NSW and had a DD risk pedigree but was not tested as the DNA test was not available at the time.

- Body wall closure defects: Midline body wall closure defects are reported in DD calves, ranging from mucocutaneous openings on the face into the nasal cavity to occipital meningocoeles, encephalocoeles and exencephaly. Spina bifida is common in DD cases and minor defects of skin closure on the dorsal midline have been reported. No cases of total rachischisis have been reported, but the rachipagus partial conjoined twin does occur in DD. Eventration of the abdominal viscera through the ventral midline has also been reported in some newborn DDA calves.


- Congenital arthrogryposis and hindlimb deformity: Some DDA calves are born with deformed hindlegs, usually with some degree of arthrogryposis, but with no other signs of DD. This abnormal development of the hindlimbs is likely to result from intrauterine hypokinesia associated with spinal cord dysfunction, either from dysraphic tethering of the cord or from cord compression by an intramedullary teratoma. The usual hindlimb conformation in these cases involves joint fixation with the hindlegs projecting forwards.

- Post-natal ataxia: Cord tethering or compression may not become clinically significant until after birth in DDA calves, sometimes well after birth. Some DDA cases have presented with a post-natal onset of progressive hindlimb ataxia but no external sign of DD. Careful palpation of the spine may disclose a tumour along the spine or scoliosis associated with dysraphism and vertebral anomalies. The cause of ataxia may however only be detected by radiology or necropsy.
- Embryonic deaths: Dysraphic syndromes in other species are associated with high rates of post-implantation embryonic death and resorption. It is considered very likely that the much of the mortality in DD that is predicted from the genotype data is occurring during embryogenesis. Research is underway to demonstrate this. In the meantime, DD should be considered as a possible cause of delayed return to service or abortion where both parents are known or likely to be DD carriers.
Other presenting signs are expected in DD based on the comparative pathology but have not yet been detected in DDA calves. It is possible that some of the duplications observed externally in DDA cases can also occur internally. An intra-abdominal leg was reported in one human dysraphic case. Based on observations from human medicine and laboratory animals, it is also expected that a proportion of congenital teratomas will become active later in life, with any expanding intracranial or intramedullary teratoma quickly becoming clinically significant. Dysraphia has also been associated with thymic aplasia and aortic root anomalies. We are continuing to survey Angus calves for further DD phenotypes.
PLANNED FURTHER RESEARCH
Suspect DD cases that match the clinical descriptions above are needed for this research - live animals or fresh cadavers. Brains, spinal cords and teratomas are the tissues of most interest, but they are difficult to remove for fixation without destroying some of the significant abnormalities. Histopathology has been limited to date by significant autolysis in the case material received.
DNA testing from EDTA blood or tail hair samples of abnormal calves that meet the case criteria above and are submitted through Dr Denholm (see contact details below) with a full history and a photograph of any external abnormalities or necropsy findings will be undertaken free of charge to the submitter until further notice.
We suggest that EDTA blood from any newborn Angus calf or aborted foetus which meets any of the phenotype descriptions above should be routinely submitted for DNA testing for DD. Prof. Beever is also seeking ongoing submission of DNA samples from all polymelia and conjoined twin cases to continue this research. DNA samples from DDA calves will be routinely forwarded to his laboratory in Illinois by Australian laboratories.
Photographs of DD cases and the anatomic pathology in this heritable disease can be viewed online at Dr Denholm's Photobucket site s107.photobucket.com.
Field veterinarians who identify suspected cases of DD are asked to contact Dr Denholm in the first instance on 0418 641957, any time of day or evening including weekends.
CONTACT:
Dr Laurence Denholm
BVSc(Hons) LLB(Hons) DipAgSc GradDipLegPrac PhD(Cornell)
Principal Policy Analyst
Strategic Policy and Economics Division
NSW Trade and Investment
Locked Bag 21 ORANGE NSW 2800 Australia
T 61 2 6391 3634 F 61 2 6391 3650 M 0418 641957
eMail laurence.denholm@trade.nsw.gov.au
|| Posted 4 December 2013 ||